Welcome to Stangen Pharmaceuticals
Philippines, Inc.
STANDARD,
ADVANCED,
GENUINE, NOVEL
First company to launch Ceftriaxone + Sulbactam and is a brand leader. Second company to launch Pregabalin.
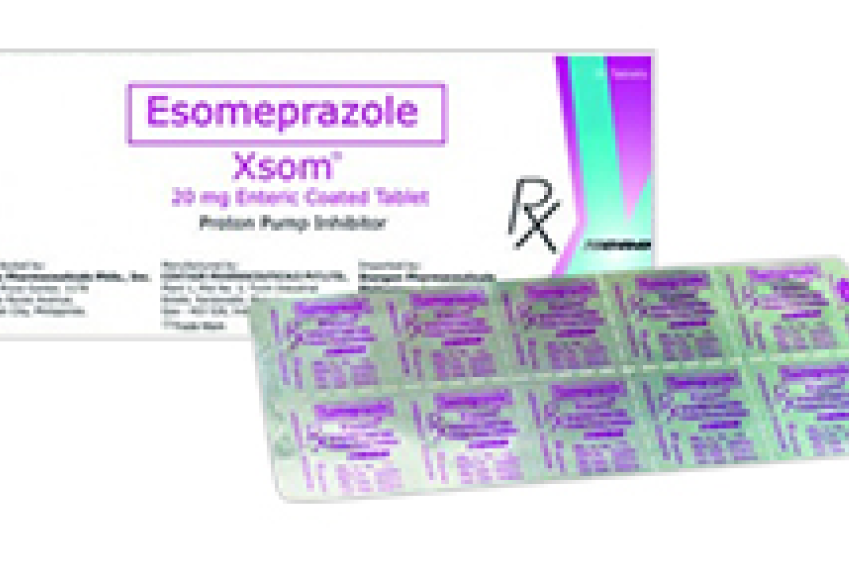
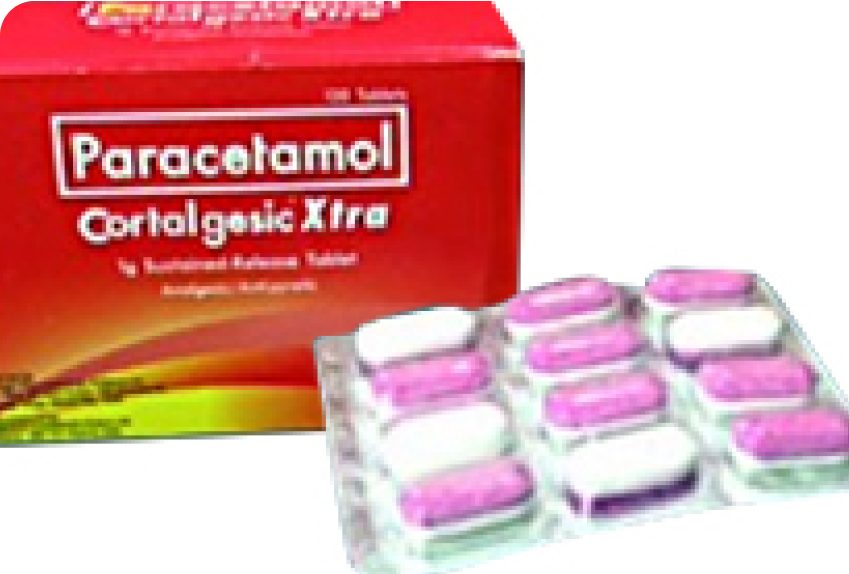
Ranked high in Celecoxib 200 Mg. Leader in Clomiphene citrate in terms of units. First company to launch Esomeprazole tablets and is a generic leader. First company to launch a unique product Paracetamol 1000 MG SR tablets.
Welcome to Stangen Pharmaceuticals Philippines, Inc.
STANDARD,
ADVANCED,
GENUINE, NOVEL
First company to launch Ceftriaxone + Sulbactam and is a brand leader. Second company to launch Pregabalin.
Ranked high in Celecoxib 200 Mg. Leader in Clomiphene citrate in terms of units. First company to launch Esomeprazole tablets and is a generic leader. First company to launch a unique product Paracetamol 1000 MG SR tablets.

Our Partners
Our Partners are approved with:

Our Partners
Our Partners are approved with:







Welcome
Stangen Pharmaceuticals was established in 2010 with an objective to introduce quality bioequivalent generic products from reputed pharmaceutical companies with transnational accreditations like USFDA, UKMHRA, TGA, PICS companies in Philippines.
Stangen stands for Standard, Genuine, Advanced and Novel that are FIRST and FAST in the market.
Stangen is engaged in importation, distribution of pharmaceuticals acquired from licensing long term agreements with renowned principals from various parts of the world and are accredited with quality embellishments across the globe.

Our Services
“Quality is what we deliver, reliability is what you get”
“Quality is what we deliver,
Reliability is what you get”

Patent Research, Assessment and Evaluation

Commercial Research

Registration of the Product as per various classifications

Warehousing

Our Partnerships







We are trusted partners of the medical fraternity distinguished by products and unmatched services.
Stangen is a company that has unparalleled skills to get into strategic partnerships with major pharmaceutical companies.
Our Services
- Patent Research, Assessment and Evaluation
- Commercial Research
- Registration of the Product as per various classifications such as “Innovator”, Monitored release, and the rest
- Warehousing